Breakthroughs in Cancer Treatment
8 min read Explore groundbreaking advances reshaping cancer treatment, from immunotherapy to precision medicine, offering hope and new possibilities. (0 Reviews)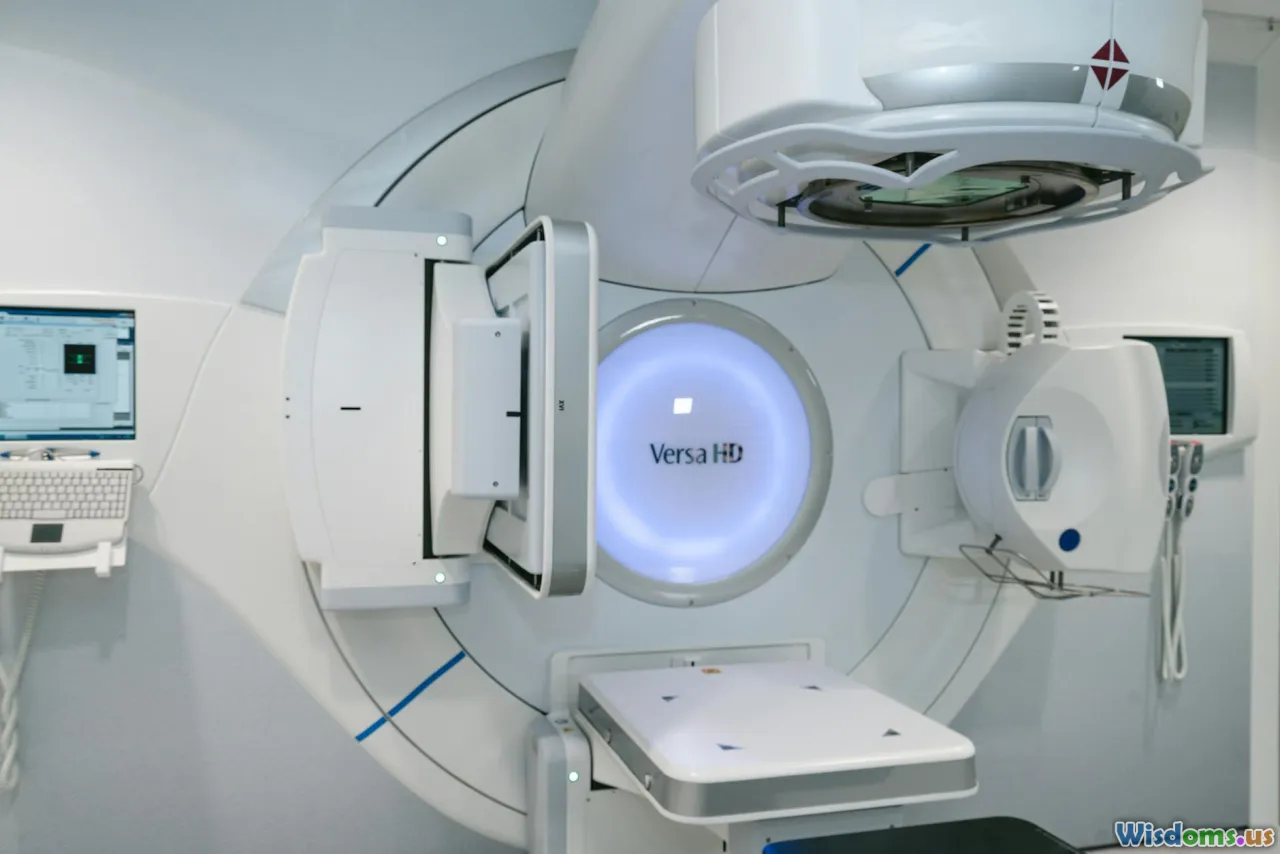
Breakthroughs in Cancer Treatment: Innovations Transforming Care
Cancer has long been one of the most complex and challenging diseases to treat. Yet in recent years, remarkable scientific and technological advances have dramatically reshaped the landscape of cancer therapy. Patients and clinicians alike stand at the threshold of a new era where innovation makes previously unimaginable outcomes possible. From immunotherapy activating the body’s own defenses to AI-driven precision medicine offering personalized treatment plans, breakthroughs in cancer treatment hold promise to drastically improve survival and quality of life.
Introduction: The Evolving Battle Against Cancer
Historically, cancer treatment relied heavily on surgery, chemotherapy, and radiation—methods effective but often accompanied by severe side effects and recurrent relapse. However, an explosion of research spanning molecular biology, genetics, technology, and immunology has refueled hope. This article delves into some of the most transformative breakthroughs shaping modern cancer care. Through concrete examples and cutting-edge clinical insights, we’ll explore how these developments not only improve outcomes but also challenge traditional approaches in oncology.
1. Immunotherapy: Harnessing the Immune System to Defeat Cancer
One of the most revolutionary advances in cancer treatment is immunotherapy, which stimulates or restores the patient’s immune system to recognize and destroy cancer cells. Unlike chemotherapy that non-selectively attacks rapidly dividing cells, immunotherapy aims for precision and durability.
Checkpoint Inhibitors
Checkpoint inhibitors such as pembrolizumab (Keytruda) and nivolumab (Opdivo) have transformed treatment for cancers like melanoma, non-small cell lung cancer, and bladder cancer. These drugs block proteins that inhibit T-cell activation (e.g., PD-1/PD-L1), enabling immune cells to attack cancer. Remarkably, long-term remission is now attainable in a subset of patients once considered refractory to all treatment.
As Nobel Laureate James Allison stated, “Harnessing the patient’s own immune system was a paradigm shift in how we think about cancer.”
CAR-T Cell Therapy
Chimeric antigen receptor T-cell therapy (CAR-T) represents an engineered cell therapy where a patient’s T-cells are modified to target specific cancer antigens. First approved in 2017 for certain leukemias and lymphomas, CAR-T has demonstrated response rates around 80-90% in otherwise treatment-resistant cases. The success has sparked ongoing research for solid tumors, which pose unique challenges like tumor microenvironment barriers.
2. Precision Medicine and Targeted Therapies
Cancer is increasingly understood as a constellation of unique molecular diseases rather than a single entity. This insight has catalyzed the rise of precision medicine, tailoring therapy to genetic mutations and biomarkers within individual tumors.
Targeted Inhibitors
Drugs such as imatinib (Gleevec) targeting BCR-ABL mutations in chronic myeloid leukemia and osimertinib for EGFR-mutated non-small cell lung cancer exemplify this approach. Targeted inhibitors disrupt key pathways in cancer cells while sparing normal tissues, minimizing collateral damage.
Liquid Biopsies
Liquid biopsies represent a groundbreaking noninvasive diagnostic tool, detecting circulating tumor DNA (ctDNA) from a simple blood test. This facilitates early detection, monitoring for minimal residual disease, and dynamic treatment adjustments in real-time—enabling truly personalized cancer care.
Case Study: The NCI-MATCH Trial
The National Cancer Institute’s MATCH trial enrolls patients based on tumor genetic abnormalities rather than cancer type, demonstrating the power of molecular profiling across cancer subtypes to guide targeted treatment.
3. Advances in Radiotherapy and Surgery
Though surgery and radiation have been traditional cancer pillars, technology invites continuous evolution.
Proton and Carbon Ion Therapy
Proton therapy offers a precise form of radiotherapy that can deposit maximum energy within tumors while sparing adjacent healthy tissue. This precision decreases side effects, particularly valuable in pediatric and brain cancers. Carbon ion therapy, less widely available but with higher biological effectiveness, shows exciting potential versus photon beams.
Minimally Invasive and Robotic Surgery
Innovations like laparoscopic and robotic-assisted surgeries have reduced recovery times and complication rates. Systems such as the da Vinci surgical robot facilitate incredible precision, enabling complex tumor resection while preserving organ function.
4. Artificial Intelligence and Digital Technology
The intersection of AI and oncology is an exciting frontier with multifaceted impacts—from diagnostics to treatment planning and drug discovery.
Enhanced Diagnostics
Machine learning algorithms can analyze imaging scans and pathology slides with remarkable accuracy, often outpacing human experts in detecting subtle malignancies. For example, Google Health’s AI model improved breast cancer detection in mammograms by reducing false positives and negatives.
Drug Development and Clinical Trials
AI accelerates drug discovery by predicting compound efficacy and toxicity faster than traditional methods. It also optimizes clinical trial design, patient recruitment, and response monitoring, making research more efficient and effective.
Personalized Treatment Plans
Combining patient data—from genomic to lifestyle—AI models support oncologists in tailoring regimens, anticipating resistance, and adjusting dosages dynamically.
5. Challenges and Future Directions
While the breakthroughs bring promise, challenges persist including high costs, access inequality, and managing immune-related side effects. Multi-disciplinary collaboration among researchers, clinicians, policymakers, and patient advocates is essential to expand benefits globally.
Encouraging ongoing directions include:
- Combining immunotherapy with targeted agents to overcome resistance
- Advances in nanotechnology for drug delivery
- Expanding affordable diagnostics and telemedicine for remote populations
- Enhancing patient-centric care focusing on quality of life
Conclusion: A New Era of Hope and Possibility
The rapid pace of innovation in cancer treatment offers unprecedented hope. Breakthroughs such as immunotherapy, molecularly targeted therapies, advanced radiotherapy, and AI-driven tools are redefining what is possible—transforming cancer from often fatal to increasingly manageable or even curable. As these technologies continue evolving, wider adoption and equitable access will be crucial to ensure that millions benefit worldwide.
This monumental progress not only advances science but also inspires patients, researchers, and healthcare providers, reminding us that with ingenuity and persistence, the trajectory of cancer can be rewritten.
References:
- National Cancer Institute: https://www.cancer.gov/about-cancer/treatment/types/immunotherapy
- Wolchok JD et al., NEJM 2017: Nivolumab in Melanoma
- Schuster SJ et al., NEJM 2017: CAR-T Therapy for lymphoma
- Google Health AI breast cancer detection research
- NCI-MATCH trial information
Rate the Post
User Reviews
Popular Posts